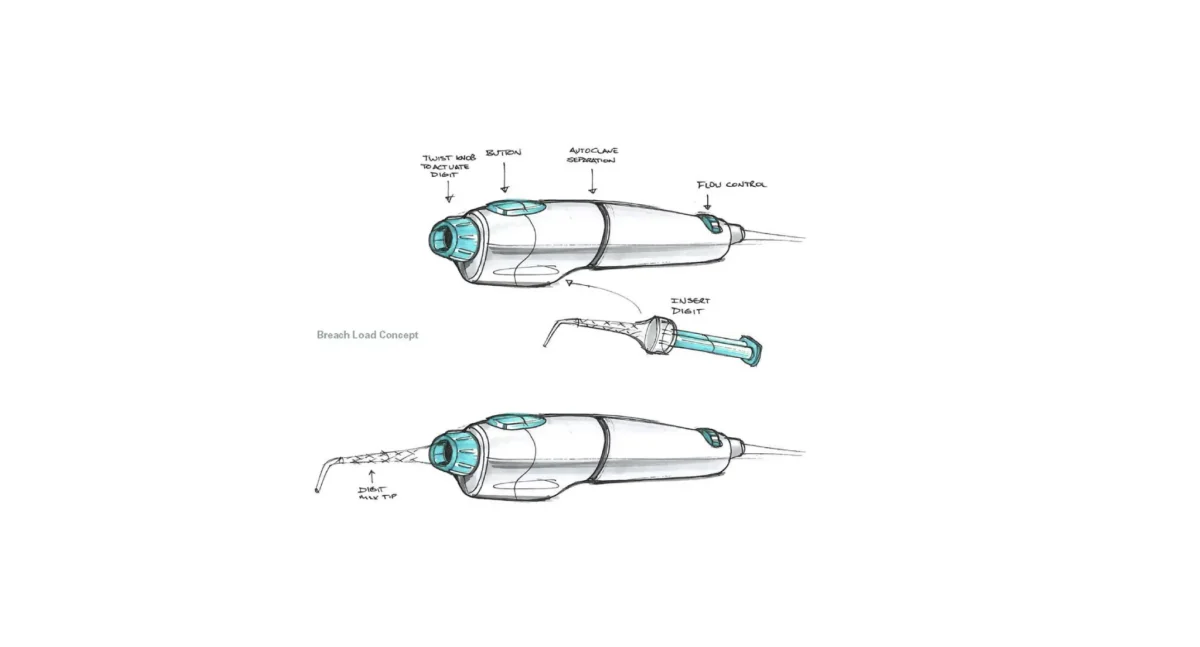
The journey of a medical device from a spark of an idea in a clinician’s mind to a life-saving tool in a patient’s hands is one of the most challenging and rewarding paths in the world of product development. At the very heart of this journey lies a critical, iterative, and fascinating process: medical device prototyping.
For anyone embarking on this path, the term “prototype” might conjure images of 3D-printed parts and rough-looking gadgets. But in the medical field, prototyping is so much more. It’s the tangible translation of a hypothesis. It’s the bridge between a theoretical solution and a practical, usable, and safe medical intervention.
At Shark Design, a product design and development company, we see prototyping not as a single step, but as the vital heartbeat of the entire innovation process. It’s how we answer difficult questions, mitigate profound risks, and ultimately, create devices that truly serve the people who use them.
This guide will walk you through the why, the how, and the what of medical device prototyping, stripping away the corporate jargon to give you a clear, human-centric understanding.
Why Prototyping Isn’t Optional in Medical Device Development?

In a sector governed by stringent regulations like those from the FDA and ISO 13485, why is building rough early versions of a device so indispensable? The reasons are multifaceted and impact every aspect of the product’s future.
1. De-risking Your Investment (and Protecting Patients)
This is the most critical reason. Medical device development is capital-intensive. A fundamental flaw discovered during late-stage clinical trials or, worse, after a product launch, can be catastrophic—both financially and for patient safety. Prototyping uncovers these flaws early and inexpensively. It’s far cheaper to revise a 3D-printed model than to retool a $100,000 injection mold.
2. Validating the Core User Experience (UX)
A medical device has two primary users: the clinician and the patient. A design that looks perfect on paper might feel awkward in a surgeon’s hand, or the controls might be confusing under the stress of a procedure. Similarly, a wearable device might be uncomfortable for a patient to use for 12 hours a day. Physical prototypes allow for real-world usability testing, gathering invaluable feedback that shapes the device into an intuitive and ergonomic tool.
3. Securing Funding and Building Stakeholder Buy-in
An idea described in a PowerPoint presentation is abstract. A working prototype that an investor can hold, or a surgeon can try, is powerful and tangible. It demonstrates commitment, proves technical feasibility, and makes the vision concrete, making it significantly easier to secure the funding needed for the next phase of development.
4. Guiding Regulatory Strategy
Engaging with regulatory bodies is a conversation. Bringing a well-considered prototype to a preliminary meeting with the FDA, for example, provides a concrete basis for discussion about testing protocols, human factors validation, and the overall path to approval. It shows you’ve moved beyond theory and are seriously considering the practical aspects of safety and efficacy.
5. Driving Engineering and Manufacturing Discovery
Prototyping forces answers to critical engineering questions. How will the parts fit together? Is the battery life sufficient? Will the plastic housing withstand repeated sterilization? Each prototype iteration refines the design for manufacturability (DFM), ensuring that when you’re ready for mass production, the transition is smooth and cost-effective.
The Medical Device Prototyping Process: A Phased Approach
Prototyping isn’t a one-and-done event. It’s a strategic, phased evolution where each stage serves a distinct purpose, increasing in fidelity and complexity as the design matures.
Phase 1: Proof-of-Concept (PoC) Prototypes
- Purpose: To answer one simple, fundamental question: “Does the core technology or mechanism work?”
- What It Looks Like: These are often “Frankenstein” devices—ugly, cobbled together from off-the-shelf components, breadboards, duct tape, and software scripts. Fidelity is zero; function is everything.
- Key Activities: Basic feasibility testing. Does the sensor detect what it should? Does the fluid move through the mechanism as intended? Does the algorithm correctly interpret the data?
Phase 2: Form and Fit Prototypes
- Purpose: To explore the physical embodiment, ergonomics, and aesthetics of the device.
- What It Looks Like: These are often non-functioning, 3D-printed models that represent the exact size, shape, and feel of the final product. They may have moving parts but no internal electronics or software.
- Key Activities: Usability testing with clinicians. How does it feel in a glove? Can buttons be reached easily? Is the display readable at a glance? This phase is all about the human-device interface.
Phase 3: Alpha / Works-Like, Looks-Like Prototypes
- Purpose: To integrate function and form, creating a high-fidelity model that behaves and appears as the final product would.
- What It Looks Like: This is where the electronics, software, and mechanics are integrated into a housing that closely resembles the final design. It may still use some commercial components, but it’s a cohesive, working unit.
- Key Activities: Comprehensive functional testing, preliminary software validation, and more advanced user feedback sessions. This is the prototype often used for early investor demos and regulatory pre-submissions.
Phase 4: Beta / Design Verification Prototypes
- Purpose: To validate the final design and build units for formal Design Verification testing.
- What It Looks Like: These units are manufactured using production-intent processes and materials. They are, for all intents and purposes, the final product, built in a pilot production environment rather than a full-scale factory.
- Key Activities: Rigorous, documented testing to prove the device meets all its predefined design inputs (performance, safety, reliability, etc.). The data generated here is submitted to regulatory bodies.
Phase 5: Pilot Production / Clinical Trial Units
- Purpose: To manufacture a small batch of devices for human clinical trials.
- What It Looks Like: These are final products, built on the planned production line or with the chosen contract manufacturer. They must be consistent, reliable, and sterile (if applicable).
- Key Activities: The devices are used in clinical studies to gather evidence of safety and efficacy in the target patient population.
Key Technologies Powering Modern Medical Prototyping
The speed and flexibility of modern prototyping are driven by several key technologies.
- 3D Printing (Additive Manufacturing): An absolute game-changer. It allows for the rapid creation of complex geometries in hours or days, not weeks. At Shark Design, we use technologies like SLA for high-detail parts and SLS for durable, functional components. It’s perfect for form-and-fit models, surgical guides, and custom jigs.
- CNC Machining: For parts that need to be made from the final production material (e.g., specific medical-grade plastics or metals), CNC machining is ideal. It offers excellent precision and material properties, making it suitable for works-like prototypes and small-batch beta units.
- Rapid Tooling & Injection Molding: For producing dozens to hundreds of high-fidelity parts in the final production plastic, rapid tooling (using aluminum molds) is the bridge between prototyping and mass production. It provides parts with the exact look, feel, and performance of the final product.
- Printed Circuit Board (PCB) Prototyping: The “brain” of many modern devices. Rapid PCB fabrication and assembly services allow us to iteratively test and refine the electronics in parallel with the mechanical design.
- Software-in-the-Loop (SIL) and Hardware-in-the-Loop (HIL): These simulation-based approaches allow us to test device software against virtual or physical representations of the hardware, catching bugs and logic errors long before full integration.
Navigating the Unique Challenges of Medical Prototyping
Medical device prototyping doesn’t happen in a vacuum. It’s shaped by a unique set of constraints that must be respected from day one.
- Regulatory Compliance: You must design with the FDA’s Quality System Regulation (QSR) and ISO 13485 in mind. This means design controls—documenting every requirement, every design change, and every test result from the very first prototype.
- Human Factors & Usability Engineering (HF/UE): Prototyping is the primary tool for fulfilling human factors requirements. It’s about proving that the device can be used safely and effectively by the intended users in the intended use environment, without error.
- Biocompatibility: Any material that contacts the patient’s body must be tested for biocompatibility (per ISO 10993). This consideration must begin at the prototype phase. You can’t test a final product made from a material you’ve never prototyped with.
- Sterilization and Durability: Will the device be autoclaved? Exposed to harsh chemicals? Dropped on the floor? Prototypes, especially beta units, must be tested to withstand the rigors of the clinical environment.
Why Shark Design Approaches Prototyping Differently
At Shark Design, we believe the magic of prototyping isn’t just in the technology; it’s in the mindset. We don’t see ourselves as just a service provider; we are your partner in de-risking your journey to market.
Our approach is built on three pillars:
- Clarity Through Tangibility: We push to get a physical representation of an idea in your hands as soon as possible. This transforms abstract discussions into concrete, actionable feedback.
- Embrace Intelligent Failure: We create a culture where prototypes are tools for learning, not just milestones to be checked off. Every “failure” discovered in our lab is a victory—a risk identified and mitigated before it could impact a patient or your budget.
- Design for the Real World: We are constantly asking the “what if” questions. What if a nurse is distracted? What if the battery is low? Our prototyping process is infused with human factors principles, ensuring the devices we help create are not just technically brilliant, but also inherently safe and easy to use.
Prototyping as Your Strategic Compass
In the high-stakes world of medical device development, prototyping is your most powerful tool for navigating uncertainty. It transforms guesswork into knowledge, reduces astronomical risks into manageable challenges, and ensures that the final product truly meets the needs of the people it was designed for.
Bottom Line
It’s a disciplined, iterative dialogue between the idea and reality. By investing in a robust, phased prototyping strategy from the very beginning, you aren’t just building a device—you are building the evidence, the confidence, and the foundation for its ultimate success.
Are you navigating the complex journey of medical device innovation? Let’s talk. At Shark Design, we’re ready to help you build your next breakthrough, one prototype at a time.